Abstract
Background: Progressive disease (PD) in MF is associated with increased bone marrow fibrosis, worsening anemia, and an increase in circulating blasts and can result in leukemic transformation (LT). LT is a rare (~11% incidence) but fatal complication of MF with a mortality rate of 97% and a median survival of 3.6 months. Real-world data regarding PD and LT in lower-risk MF are limited. This analysis from the Myelofibrosis and Essential Thrombocythemia Observational Study (MOST; NCT02953704) describes the incidence of PD, including LT, in patients with lower-risk MF and compares the baseline characteristics of patients who progressed vs those who did not.
Methods: MOST is a longitudinal, noninterventional, prospective, observational study of patients with clinical diagnoses of MF or essential thrombocythemia enrolled at clinical practices in the US. Patients included in this analysis (≥18 years) had low-risk or intermediate-1 (INT-1)-risk MF (by age >65 years alone) stratified by the Dynamic International Prognostic Scoring System (Blood. 2010;115:1703) at enrollment and were followed for at least 3 years. Baseline data were collected as previously described (Gerds A. Clin Lymphoma Myeloma Leuk. 2022;22:e532-e540). PD was defined as physician-reported PD, LT, and/or death by reason of progression.
Results: Of the 232 patients with MF enrolled in MOST, 204 patients (low-risk: n=84, 41.2%; INT-1-risk: n=120, 58.8%) were included in this analysis (data cutoff: July 5, 2022). The median (range) time from MF diagnosis to enrollment was 1.8 (0-38) years and the median (range) enrollment duration was 4.2 (3.3-5.4) years.
Of 193 patients with available data on disease progression, 46 patients (23.8%) had physician-reported PD; of these, 18 (22.0%) were low-risk and 28 (25.2%) were INT-1-risk. The most commonly reported indicator was change in hematologic parameters (n=27 [58.7%]), followed by change in spleen size (n=19 [41.3%]), MF-related symptoms (n=16 [34.8%]), and blast counts (n=8 [17.4%]). LT was reported in 15 patients (7.8%); of these 4 (4.9%) were low-risk and 11 (9.9%) were INT-1-risk. Death by any cause was reported in 30 patients (14.7%; PD, n=16, without PD, n=9; unknown PD, n=5).
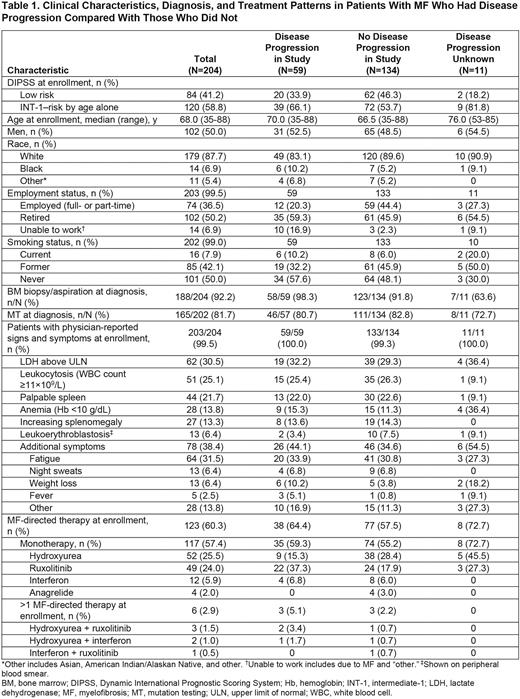
Patient baseline characteristics are shown in Table 1. Patients who had PD were older than patients without (median [range] age: PD, 70.0 [35-88] vs without PD, 66.5 [35-88] years). Bone marrow biopsy/aspiration and mutation tests at diagnosis were reported in 98.3% and 80.7% of patients with PD and in 91.8% and 82.8% of patients without PD, respectively. Significant differences in the symptomatology of patients with PD vs without PD were not detected at time of enrollment. More than half of patients with PD and without PD were receiving MF-directed monotherapy at enrollment (59.3% vs 55.2%). The most common monotherapies received in patients with PD vs without PD were hydroxyurea (15.3% vs 28.4%) and ruxolitinib (37.3% vs 17.9%). Six patients (PD, n=3; without PD, n=3) received >1 MF-directed therapy.
Conclusion: This real-world analysis of data from patients enrolled in MOST describes the disease evolution of patients with lower-risk MF. After ~3 years of follow-up, a relatively high proportion of patients in this population had physician-reported PD or LT (23.8% and 7.8%, respectively). Baseline characteristics and symptoms did not differ between those who progressed and those who did not; however, further analysis of molecular and post-baseline data may provide insight into risk factors for disease progression in this lower-risk population. Identification of patients with higher risk of PD is likely to improve disease management and outcomes for patients with lower-risk MF.
Disclosures
Komrokji:AbbVie: Consultancy, Honoraria, Speakers Bureau; Acceleron Pharma: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Consultancy, Honoraria, Speakers Bureau; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; CTI BioPharma, Innovent: Honoraria, Membership on an entity's Board of Directors or advisory committees; Geron: Consultancy; PharmaEssentia, Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Taiho Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Servier: Consultancy, Honoraria, Speakers Bureau. Grunwald:Genetech/Roche, Incyte Corporation, Janssen: Research Funding; Medtronic: Current equity holder in private company; Daiichi Sankyo, Gamida Cell, Gilead Sciences, Incyte Corporation, Invitae, Karius, Novartis, Ono Pharmaceutical, Pfizer, Pharmacosmos, Premier, Sierra Oncology, Stemline Therapeutics: Consultancy; AbbVie, Agios/Servier, Amgen, Astellas Pharma, Blueprint Medicines, Bristol Myers Squibb, Cardinal Health, CTI BioPharma, Daiichi Sankyo, Gamida Cell, Gilead Sciences, Incyte Corporation, Invitae, Karius, Novartis, Ono Pharmaceuticals, Pfizer, ,: Consultancy. Braunstein:Incyte Corporation: Current Employment, Current equity holder in publicly-traded company. Hamer-Maansson:Incyte Corporation: Current Employment, Current equity holder in publicly-traded company. Kalafut:Incyte Corporation: Current Employment, Current equity holder in publicly-traded company. Mascarenhas:Imago: Consultancy; Merck: Research Funding; CTI BioPharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Consultancy; Celgene/BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sierra Oncology: Consultancy; GSK: Consultancy; Merus: Research Funding; Forbius: Research Funding; AbbVie: Consultancy, Research Funding; Kartos: Consultancy, Research Funding; Geron: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Galecto: Consultancy; PharmaEssentia: Consultancy, Research Funding; Roche: Consultancy, Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janseen: Research Funding; Prelude Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal